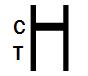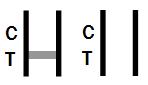Medical Diagnostic Dengue NS1 Test kit , rapid test
ASSAY PROCEDURE
Step 1: Bring the specimen and test components to room temperature if refrigerated or frozen. Mix the specimen well prior to assay once thawed.
Step 2: When ready to test, open the pouch at the notch and remove device. Place the test device on a clean, flat surface.
Step 3: Be sure to label the device with specimen’s ID number.
Step 4: For whole blood sample:
Fill the dropper with the specimen then add 2 drops(App.50µL)of specimen into the sample well. Making sure that there are no air bubbles.
Then add 2 drops of Sample Diluent immediately into the sample well.
For Plasma/ Serum sample:
Fill the dropper with the specimen then add 1 drop(App.25µL) of specimen into the sample well. Making sure that there are no air bubbles.
Then add 2 drops of Sample Diluent immediately into the sample well.
Step 5: Set up a timer.
Step 6: Read the result at 10 minutes.
Don’t read results after 30 minutes. To avoid confusion, discard the test device after interpreting the result.
INTERPRETATION OF ASSAY RESULT
|
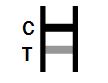
POSITIVE RESULT: |
Two colored bands appear on the membrane. One band appears in the control region (C) and another band appears in the test region (T). |
|
NEGATIVE RESULT: |
Only one colored band appears in the control region (C). No apparent colored band appears in the test region (T). |
|
INVALID RESULT: |
Control band fails to appear. Results from any test which has not produced a control band at the specified reading time must be discarded. Please review the procedure and repeat with a new test. If the problem persists, discontinue using the kit immediately and contact your local distributor. |
INTENDED USE
The Dengue NS1 Rapid Test Device is a lateral flow chromatographic immunoassay for the qualitative detection of dengue virus antigen (Dengue Ag) in human whole blood, serum or plasma. It is intended to be used as a screening test and as an aid in the diagnosis of infection with Dengue viruses. Any reactive specimen with the Dengue NS1 Rapid Test Device must be confirmed with alternative testing method(s) and clinical findings
Company Advantage
1.Recognized as a high-tech enterprise in China,a number of applications for patents and software copyrights have been approved
2.Deliver goods as order request
3.ISO13485, CE, Prepare various shipping documents
4.Reply customers questions within 24 hours